I had written a piece yesterday that I was going to publish this morning. I had to scrap most of it because for Josh Hardy, he now has a chance to survive cancer.
My original piece condemned a pharma company of being heartless bastards after reading about a boy whose last hope for survival was an experimental drug that the company could, but would not provide. Their stance had social media sites buzzing with negative comments about the company and bloggers spreading the word about them.
Through it all, the pharma company was standing firm. They insisted that if they provided the drug to the boy they would have to provide the drug to everyone who wanted it, and that would delay approval. It would also cost them a lot of money.
Ultimately, the combined pressure of social media and bad press that the pharma company was receiving turned the tide late yesterday.
The North Carolina pharmaceutical company has reversed its stance and announced it will provide a cancer-stricken 7-year-old with an experimental drug after public outrage condemned a policy that forbid them to give him the potentially life-saving drug.
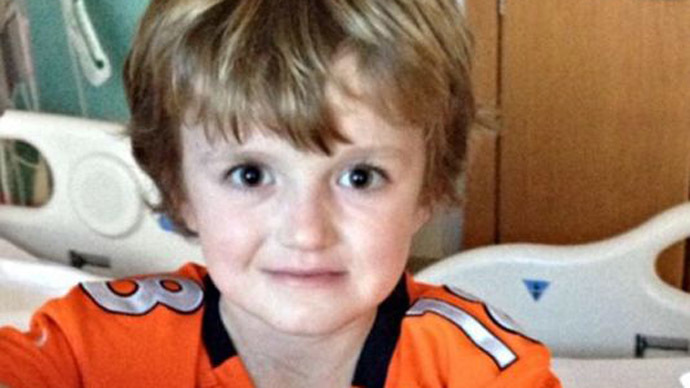
Josh Hardy has been battling various forms of cancer since he was only nine months old. Whether it was in his kidneys, thymus, lungs, or bone marrow, the boy managed to overcome the disease every time.
After a bone marrow transplant severely damaged what remained of his immune system, though, doctors discovered in February that a viral infection currently spreading through his body is putting his life in danger. As noted by CNN, he is currently in critical condition and vomits blood multiple times every hour.
The attending doctor told Todd Hardy, Josh’s father, there is one drug that “will cure and save” the boy, currently being developed by North Carolina-based Chimerix. Despite “multiple requests” made by St. Jude’s Children Research Hospital in Memphis, Tennessee, however, the company has declined the provide the medication.
“Our son will die without this drug,” Todd Hardy told CNN. “We’re begging them to give it to us.”
When asked why the company won’t supply the antiviral called brincidofovir, Chimerix President and CEO Kenneth Moch said that if it did for one patient, it would have to do it for all the others who request it.
“We have have great compassion for this family,” Moch said to USA Today. “But this is not just about a single boy.”
“If this were just one patient wanting this drug, then this would be a very different question,” he added to CNN. “But it’s yes to all or no to all.”
The Hardys’ plight inspired Internet users from all over the world to use social media to pressure the company to change its stance. In a statement Tuesday, Chimerix revealed, the pressure worked – Josh will be one of 20 patients to receive an experimental clinical trial of the drug. While the Food and Drug Administration has yet to approve the drug, the agency did grant Chimerix permission to carryout the trial.
Josh’s family is staying with him at St. Jude Children’s Research Hospital in Memphis, Tennessee. From there they thanked supporters via Facebook.
“You did it. You Saved Josh. Thank you Chimerix and Josh’s Army,” they wrote.
While the experimental drug doesn’t guarantee the boy will be cured, it does provide him a better chance than he had without it.
Disclaimer: On January 4, 2016, the owner of WestEastonPA.com began serving on the West Easton Council following an election. Postings and all content found on this website are the opinions of Matthew A. Dees and may not necessarily represent the opinion of the governing body for The Borough of West Easton.







